Table of Content

Clinical Trial Project Managers ensure the smooth execution of clinical trials, from planning to completion. They manage teams, budgets, and regulatory compliance, keeping trials on track and within scope.
Their expertise is important for meeting deadlines, reducing costs, and ensuring the success of medical research. In this blog, discover the key responsibilities of a Clinical Trial Project Manager and their crucial role in advancing healthcare.
Table of Contents
1) Who is a Clinical Trial Project Manager?
2) What Does a Clinical Trial Project Manager do?
3) Responsibilities of Clinical Trial Project Manager
4) Importance of Clinical Trail Project Managers
5) Conclusion
Who is a Clinical Trial Project Manager?
A Clinical Trials Project Manager directs, implements, coordinates, controls, and executes global-scale clinical projects. They oversee the progress of clinical trials, ensuring smooth execution throughout the project.
Their role includes supporting the project planning phase and maintaining overall trial direction. They also ensure key milestones are completed on time, to a high standard, and within required guidelines, keeping the trial aligned with regulatory and quality standards.
Responsibilities of Clinical Trial Project Manager
Clinical Trial Project Managers have many responsibilities. They can differ based on the business and projects they work on. However, there are common tasks they share.
All Clinical Trial Project Managers lead clinical studies. They support the planning, setup, and coordination of clinical trials. This can include trials on medical devices, medicines, or nutritional products.
Other responsibilities include:
1) Developing and managing budgets
2) Ensuring trials follow industry guidelines and legislation
3) Identifying and mitigating potential trial risks
4) Supporting patient eligibility checks
5) Resolving protocol deviation issues
6) Acting as the main contact for sponsors and project teams
7) Assisting in preparing Clinical Study Reports (CSRs)
8) Reporting on clinical trial progress
9) Negotiating with suppliers
10) Supporting the creation of clinical trial proposals
11) Helping with the general running of clinical trials and resolving any issues
Want to lead projects with agility? Join our Agile PM® Foundation and master Agile Methodologies for success.
Importance of Clinical Trail Project Managers
Clinical Trial Project Managers are vital for smooth and successful clinical trials in the CRO space. Their role is becoming more important as the market evolves. Clinical trials were essential in overcoming the COVID-19 pandemic.
The rapid approval of vaccines has changed how these trials are conducted. Faster and more detailed trials are now a big challenge that Clinical Trial Project Managers can help overcome.
There are three main reasons why Clinical Trial Project Managers are essential:
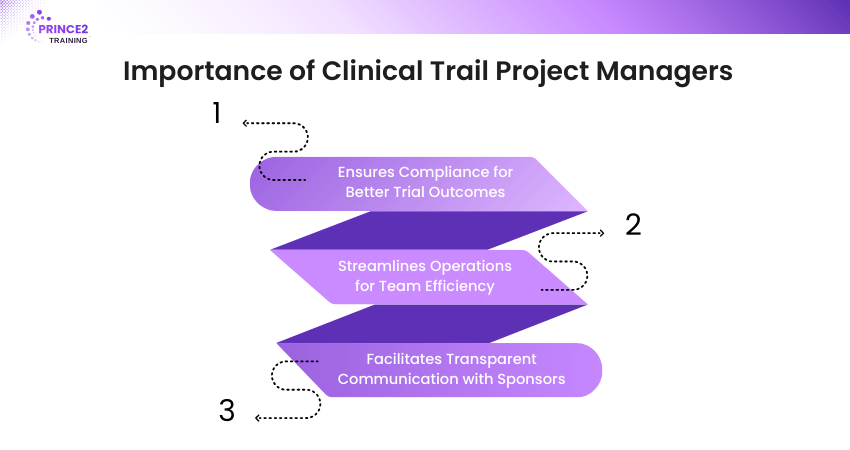
1) They ensure best practices and legislation are followed, strengthening the trial and its results.
2) They facilitate smooth operations, allowing the project team to focus on their expertise.
3) They ensure clear communication between trial sponsors and project teams. This guarantees transparency and continuous support.
Transform your career with our Project Management Courses designed for success -Register now!
Conclusion
Clinical Trial Project Managers are professionals who ensure clinical trials run smoothly and efficiently. Their ability to manage teams, budgets, and timelines is important for advancing medical research and bringing new treatments to patients. Without their expertise, clinical trials would face delays and potential failures.
Elevate your Project Management expertise with our Project Management Professional (PMP)® Certification and become a leader in your industry.
Frequently Asked Questions
What do you Need to be a Clinical Project Manager?
To become a Clinical Project Manager, you need a bachelor's degree in life sciences or a related field, along with experience in clinical trials or Project Management. Strong leadership, organisational, and communication skills are essential, along with knowledge of regulations like GCP (Good Clinical Practice).
What is the Highest Salary for a Clinical Trial Manager?
The highest salary for a Clinical Trial Manager can vary by location and experience, but in the UK, top professionals can earn around £90,000 annually. Factors such as certifications, industry, and years of experience influence earning potential.
What is the Clinical Trial Management Process?
The clinical trial management process includes planning, initiation, execution, monitoring, and closing of clinical trials. It involves selecting trial sites, recruiting participants, managing budgets, ensuring regulatory compliance, monitoring progress, and reporting results, all while adhering to Good Clinical Practice (GCP) guidelines.
 Contact@prince2training.co.uk
Contact@prince2training.co.uk 01344203999
01344203999





 Back
Back







 Continue Browsing
Continue Browsing
